Abstract
INTRODUCTION: The myeloid differentiation antigen CD33 is an established therapeutic target in acute myeloid leukemia (AML). In addition to its broad expression on leukemic cells of most patients with AML, the endocytic properties of the receptor make it well suited for antibody-drug conjugate (ADC)-based strategies. IMGN779 is a next-generation anti-CD33 ADC with a novel DNA-alkylating IGN (indolinobenzodiazepine pseudodimer) payload and a cleavable s-SPDB linker, which entered clinical evaluation based on potent preclinical antitumor activity and superior tolerability compared with DNA-crosslinking payloads. We report safety and antileukemia activity findings from the ongoing dose escalation study of IMGN779 in patients with relapsed or refractory AML.
METHODS: The objectives of this Phase I study (NCT02674763) include determination of the dose-limiting toxicity (DLT), safety, pharmacokinetics (PK), pharmacodynamics (PD), and preliminary antileukemia activity of IMGN779. Adult patients with relapsed or refractory CD33+ AML (defined as ≥ 20% of blasts expressing CD33 by flow cytometry) are eligible. IMGN779 is administered intravenously and dose escalation follows a standard 3+3 design. Two schedules have been evaluated to date: once every 2 weeks (Q2W; i.e. Days 1 and 15 of a 28-day cycle) and once weekly (QW; Days 1, 8, 15, and 22 of a 28-day cycle).
RESULTS: Fifty patients, median age 68 years (range: 27-84), have received IMGN779, with 36 and 14 patients treated on the Q2W and QW schedules, respectively. Sixteen patients (32%) had primary refractory AML, with 39 (78%) having received prior intense frontline therapy, including stem cell transplant in nine (18%) patients. Dosing using the Q2W schedule commenced at 0.02 mg/kg and has been escalated to 1.5 mg/kg (cohort 11). QW dosing was initiated at 0.39 mg/kg and escalation has proceeded to 0.7 mg/kg (cohort 3). All patients received premedication with steroids.
No DLTs have been observed on either schedule. The most commonly observed treatment-emergent AEs seen across both cohorts combined (>20% of patients) were febrile neutropenia, epistaxis, nausea, diarrhea, fatigue, abdominal pain, and hypokalemia. The most frequent Grade 3+ adverse events (AEs) were febrile neutropenia (40%), bacteremia (22%), pneumonia (20%), and anemia (16%). Three patients dosed on the QW schedule discontinued due to an AE. These involved individual cases of grade 3 diverticulitis, grade 4 large intestinal perforation, and grade 4 septic shock; none were considered related to study drug. With a median of 3.5 doses (range: 1-34) administered per patient, there has been no evidence of cumulative toxicity, nor have any correlations been observed between increasing dose and AE frequency, nature, or severity. There have been no study drug-related deaths.
For both schedules, dose-dependent increases in Cmax and AUC with prolonged exposure have been observed. A slower elimination phase was observed with higher IMGN779 doses (>0.39 mg/kg) consistent with non-linear pharmacokinetics. CD33 saturation is prolonged in a dose dependent manner and weekly dosing maintains CD33 receptor saturation.
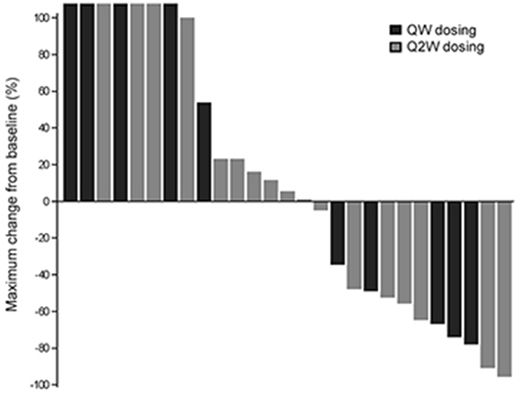
Overall, 19 of 24 patients with measurable circulating blasts (79%) showed decreases in blast numbers in the first week of therapy. Figure 1 shows the maximal changes in bone marrow blasts from baseline for all evaluable patients treated at 0.39 mg/kg or above across both dosing schedules. In total, 11/27 patients (41%) who received IMGN779 had a >30% reduction in bone marrow blasts, which included 6/17 (35%) patients in Q2W cohorts 6-11 (0.39-1.5 mg/kg) and 5/10 patients (50%) in QW cohorts 1-2 (0.39-0.54 mg/kg).
CONCLUSIONS: IMGN779 continues to display manageable tolerability and antileukemia activity in patients with relapsed or refractory AML, characterized by an adverse event profile that is consistent with the underlying disease and/or comorbidity. PK exposures and PD CD33 saturation continue to increase with dose and support further escalation of both Q2W and QW dosing schedules, which is ongoing.
Figure 1. Maximum percent changes in bone marrow blasts from baseline. Patients who received IMGN779 on the QW schedule are shaded black; those on the Q2W schedule are shaded gray.
Cortes:Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Arog: Research Funding. DeAngelo:Amgen: Consultancy; Takeda: Honoraria; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria; Blueprint Medicines: Honoraria, Research Funding; Glycomimetics: Research Funding; Pfizer Inc: Consultancy, Honoraria; Shire: Honoraria; Incyte: Consultancy, Honoraria; ARIAD: Consultancy, Research Funding; BMS: Consultancy. Erba:Janssen: Research Funding; Novartis: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Janssen: Research Funding; Immunogen: Consultancy, Research Funding; Amgen: Research Funding; Astellas: Research Funding; Amgen: Research Funding; Novartis: Consultancy, Speakers Bureau; Pfizer: Consultancy, Other: grant; Immunogen: Consultancy, Research Funding; Astellas: Research Funding; Takeda/Millenium: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Novartis: Consultancy, Speakers Bureau; Seattle Genetics: Consultancy, Research Funding; Pfizer: Consultancy, Other: grant; Celgene: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; MacroGenics: Consultancy; Amgen: Research Funding; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Daiichi Sankyo: Consultancy, Research Funding; Astellas: Research Funding; Takeda/Millenium: Research Funding; Astellas: Research Funding; Seattle Genetics: Consultancy, Research Funding; Amgen: Research Funding; Jazz: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; MacroGenics: Consultancy; Juno: Research Funding; Pfizer: Consultancy, Other: grant; Juno: Research Funding; Pfizer: Consultancy, Other: grant; Agios: Consultancy, Speakers Bureau; Agios: Consultancy, Speakers Bureau; Seattle Genetics: Consultancy, Research Funding; Incyte: Consultancy, Speakers Bureau; Seattle Genetics: Consultancy, Research Funding; Incyte: Consultancy, Speakers Bureau; Takeda/Millenium: Research Funding; Jazz: Consultancy, Speakers Bureau; Takeda/Millenium: Research Funding; Immunogen: Consultancy, Research Funding; MacroGenics: Consultancy; Jazz: Consultancy, Speakers Bureau; MacroGenics: Consultancy; Celgene: Consultancy, Speakers Bureau; Juno: Research Funding; Immunogen: Consultancy, Research Funding; Juno: Research Funding; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Celgene: Consultancy, Speakers Bureau; Agios: Consultancy, Speakers Bureau; Daiichi Sankyo: Consultancy, Research Funding; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Agios: Consultancy, Speakers Bureau; Daiichi Sankyo: Consultancy, Research Funding; Incyte: Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau. Traer:ImmunoGen: Consultancy; Tolero: Consultancy; Notable Labs: Equity Ownership. Papadantonakis:Agios pharmaceuticals: Honoraria, Other: advisory board. Blum:Forma: Research Funding; Pfizer: Consultancy; Astellas: Consultancy; Xencor: Research Funding; Boehringer Ingelheim: Research Funding; Tolero: Research Funding. Sloss:ImmunoGen: Employment. Culm-Merdek:ImmunoGen: Employment. Wang:Novartis: Speakers Bureau; Amgen: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Speakers Bureau; Novartis: Speakers Bureau; Jazz: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.